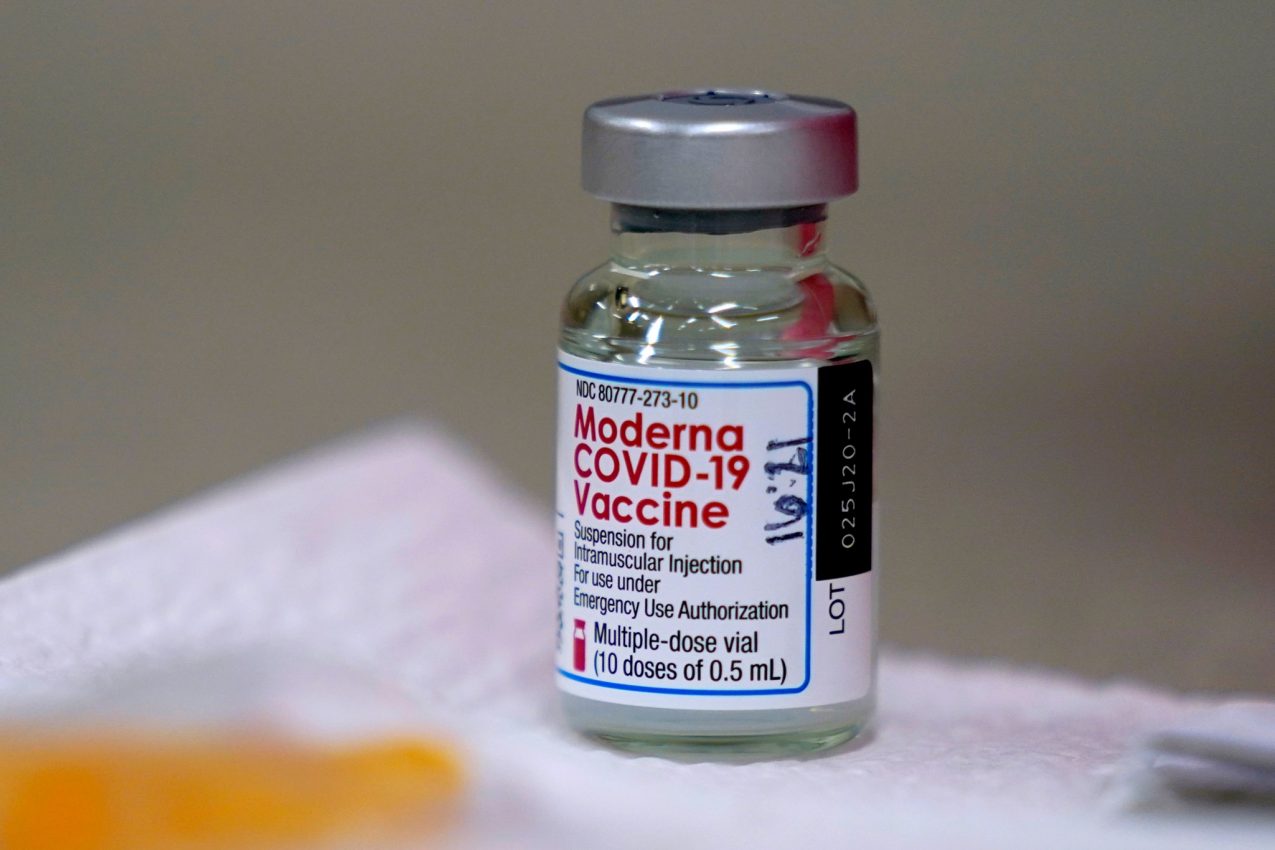
The Covid-19 vaccine developed by US biotech firm Moderna has received approval for use in the UK, becoming the third approved vaccine.
The Moderna Covid-19 vaccine works similarly with the Pfizer-BioNTech vaccine, which is currently available on the UK National Health Service (NHS). The other vaccine already approved for use in the country is from AstraZeneca.
The UK government ordered an additional 10 million doses of the Moderna vaccine, bringing the total to 17 million doses, but they are not expected to arrive until spring. A total of 367 million doses of vaccines have been ordered by the UK.
So far, about 1.5 million people in the UK have already received at least one dose of coronavirus vaccine.
Vaccine approval
Following the announcement of Moderna's approval, Health and Social Care Secretary Matt Hancock said: "This is further great news and another weapon in our arsenal to tame this awful disease."
"The NHS is pulling out all the stops to vaccinate those most at risk as quickly as possible, with over 1,000 vaccination sites live across the UK by the end of the week to provide easy access to everyone, regardless of where they live," said vaccine deployment minister Nadhim Zahawi.
The minister added: "The Moderna vaccine will be a vital boost to these efforts and will help us return to normal faster."
Moderna coronavirus vaccine
Similar to Pfizer's vaccine, the Moderna one is an RNA vaccine which injects part of the virus's genetic code in order to induce an immune response. Shipment of the vaccine requires temperatures of around -20C, similar to a regular freezer.
This is in comparison with Pfizer's vaccine which needs temperatures closer to -75C, making it more difficult to transport. Meanwhile, the AstraZeneca vaccine is considered easier to store and distribute, as it can be stored at normal fridge temperature.
The Moderna vaccine has a 94.5% effectiveness based on clinical trial results. The trial registered 30,000 participants in the US, with half being given two doses of the vaccine, four weeks apart. The rest took dummy injections. The analysis was taken from the first 95 to manifest Covid-19 symptoms.
Based on data released by the US Food and Drug Administration (FDA), the Moderna vaccine side effects include fatigue, headaches, and muscle pain as well as nausea, vomiting, and facial swelling and are likely triggered by the shots.
The data suggests the doses were generally better tolerated by people over 64 than for younger people.
The side effects of vaccines are common as they constitute an immune response that affirms the shots are working as intended, according to doctors. Many of them are warning the public to expect stronger-than-usual side effects from the Covid-19 shots than a regular flu shot.
Moderna Covid-19 vaccine production is expected to increase by 20% in 2021, according to the company’s announcement.
Moderna is aiming to manufacture up to 1 billion doses of its coronavirus vaccine this year. The U.S. will secure 100 million shots of Moderna’s vaccine by the end of March and 100 million more by June, the drugmaker said in a statement.
According to Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, the announcement "holds the promise to alter the course of this pandemic in the United States."
"With science guiding our decision-making, the available safety and effectiveness data support the authorization of the Pfizer-BioNTech COVID-19 Vaccine because the vaccine’s known and potential benefits clearly outweigh its known and potential risks," Dr. Marks said.